On Thursday, February 23, 2017, the Ohio Medical Marijuana Advisory Committee notified the public that the Cultivator rules had been filed with the Joint Committee on Agency Rule Review (“JCARR”) and published proposed rules for Testing Laboratories and Form and Method, which could make Ohio one of the most stringent states with regard to 90-day supply limitations.
Cultivator Rules
The Cultivator rules were submitted to JCARR on February 17, 2017 after review by the Common Sense Initiative Office. JCARR now has 65 days, until April 23, 2017, to review. If approved, the Department of Commerce can file the rules with the Electronic Rule Filing System and the effective date of the Rules could be as early as May 3, 2017. A copy of the JCARR rule-filing fact sheet can be found
here.
A copy of the rules as filed with JCARR can be found
here.
Testing Laboratory Rules
The proposed Testing Laboratory rules can be found
here.
Form and Method Rules
The Form and Method rules notably established the following limits for 90-day marijuana supply:
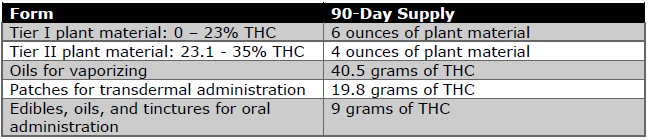
This table is very interesting. Not only does it have some of the most stringent limits nationwide, it also focuses the limits on tetrahydrocannabinol (“THC”), the psychoactive component, rather than the possible medicinal components, such as cannabidiol (“CBD”).
Thus, it appears that Ohio will regulate and define “medical marijuana” by referral only to THC. In our opinion, and, although much more research needs to be done, THC has shown mostly palliative benefits, such as reducing pain and anxiety. Conversely, CBD has shown, for example, the ability to help prevent seizures and treat people suffering from epilepsy, reduce psychotic symptoms, reduce inflammation, and prevent convulsions. Perhaps the regulatory focus will evolve over time.